satricabtagene autoleucel(Satri-cel,CT041) is an autologous CAR T-cell product candidate against the protein Claudin18.2 that has the potential to be first-in-class globally. Satri-cel targets Claudin18.2 positive solid tumors with a primary focus on gastric cancer/gastroesophageal junction cancer (GC/GEJ) and pancreatic cancer (PC).
The final follow-up results of the investigator-initiated trial CT041-CG4006 (NCT03874897) of satricabtagene autoleucel have been published in Nature Medicine on June 3, 2024.
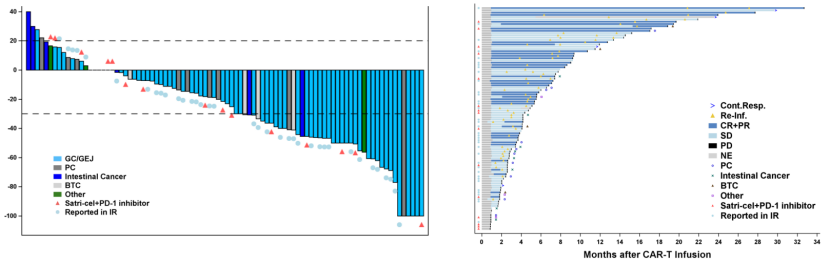
A total of 98 patients participated in the trial, and the results showed that the disease control rate (DCR) was as high as 91.8%, 38 patients achieved remission, the overall remission rate was 38.8%, the median progression free survival (PFS) was 4.4 months, the median overall survival (OS) was 8.8 months, and the median duration of remission (mDOR) was 6.4 months. Among the 90 baseline target lesion patients, 70 patients achieved varying degrees of tumor shrinkage.
Among 51 gastric cancer patients treated with monotherapy with Satri-cel, the disease control rate was as high as 96.1% (49), and the overall response rate was 54.9% (28). Among them, 27 patients achieved partial response (PR), 1 patient achieved complete response (CR), and the median duration of response (mDOR) was 6.4 months.
Conclusion
Satri-cel demonstrates therapeutic potential with a manageable safety profile in patients with CLDN18.2-positive advanced gastrointestinal cancer.
On September 9, 2023, two cases of the treatment of metastatic pancreatic cancer with CARsgen’s CT041, an innovative Claudin (CLDN) 18.2 CAR T-cell Therapy, were published in Journal of Hematology & Oncology titled “CT041 CAR T cell therapy for Claudin18.2 positive metastatic pancreatic cancer” .
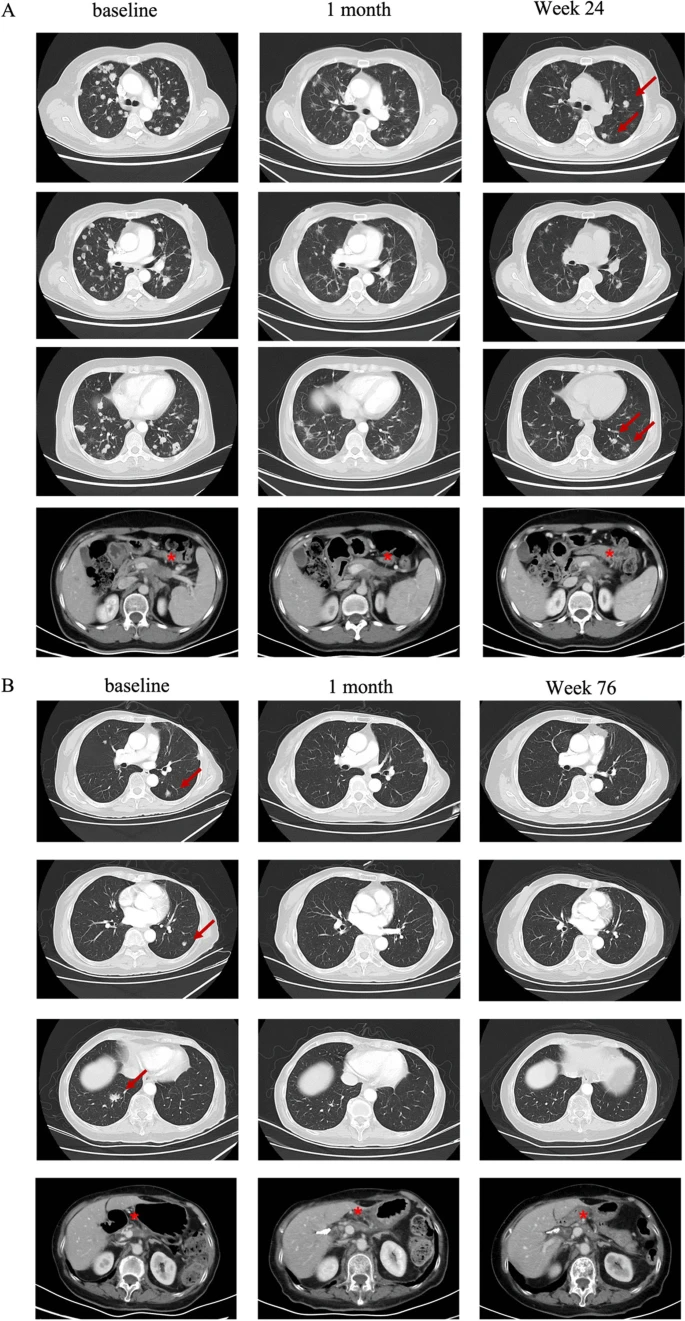
For case 1, a 58-year-old woman was diagnosed with pancreatic cancer with lung and lymph nodes metastasis. First-line nab-paclitaxel plus gemcitabine and second-line irinotecan-liposome plus 5-flurouracil were failed before she was enrolled in CT041 clinical trial, after confirming CLDN18.2 expression as 2+/70%. After lymphodepletion consisting of fludarabine, cyclophosphamide, and nab-paclitaxel, a CT041 dose of 250 × 106 cells was administered to the patient in September 2021. Grade 2 cytokine release syndrome (CRS) occurred, which was later controlled by tocilizumab. Partial response (PR) was achieved according to RECIST v1.1, with great shrinkage of lung metastasis.
For case 2, a 75-year-old woman was diagnosed as pT2N0 pancreatic cancer after she underwent surgery in May 2019. Lung metastasis was found after 5 months during routine post-surgery follow-up. S-1 monotherapy was given as the first-line chemotherapy starting from December 2019. During the surgical area palliative radiation, tumor progression was observed in the lung. Since the CLDN18.2 expression was determined as 3+/60%, the patient was enrolled in CT041 clinical trial. After receiving lymphodepletion consisting of fludarabine, cyclophosphamide and nab-paclitaxel, the patient was infused with a CT041 dose of 250 × 106 cells in July 2021. Patient experienced grade 2 CRS, which was further controlled with tocilizumab. PR was reached since the first evaluation 4 weeks after infusion. Target lesions of lung metastasis subsequently disappeared and achieved complete response. The tumor was still under well control until the last follow-up in July 2023. The peripheral blood CLDN18.2 CAR copy number in both patients exhibited a rapid post-infusion increase, peaking within 2 weeks. Concurrently, fluorescence-activated cell sorting results indicated an increase in peripheral blood CD8 T cells and Treg cells, alongside a reduction in CD4 T cells and B cells. These shifts in immune cell phenotypes demonstrated greater persistence compared to alterations in cytokine levels.
At present, there are many other CAR-T clinical trials in China, and they are looking for patients. For consultation on new drugs and technologies, you can contact Beijing South Region Oncology Hospital International Department.
Phone Number:4008803716
Email:myimmnet@163.com
References
1.https://www.carsgen.com/en/news/carsgen-presents-updated-results-on-satri-cel-in-nature-medicine-and-at-2024-asco/
2.https://www.nature.com/articles/s41591-024-03037-z
3.https://www.carsgen.com/media/fooloi3a/ct041-cg4006-asco-2024_v3-0_20240603.pdf
4.https://jhoonline.biomedcentral.com/articles/10.1186/s13045-023-01491-9
Post time: Nov-21-2024