On February 21, 2025, the Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) has granted Breakthrough Therapy Designation (BTD) for Satri-cel(CT041)(an autologous CAR T-cell product candidate against Claudin18.2) ,for advanced gastric/esophagogastric junction (G/GEJ) adenocarcinoma with positive expression of Claudin18.2 (CLDN18.2) that has previously failed at least second-line treatment.
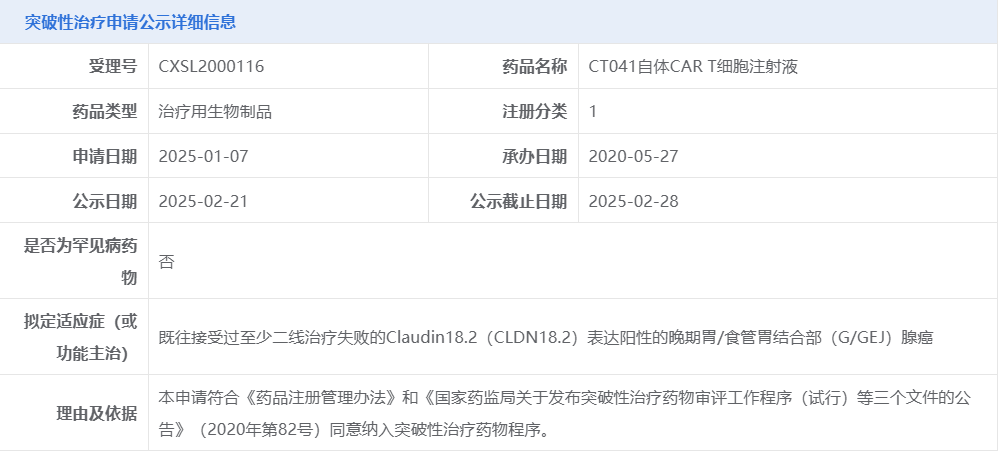
The investigator-initiated trial CT041-CG4006 (NCT03874897) of satricabtagene autoleucel (“satri-cel”, CT041) (an autologous CAR T-cell product candidate against Claudin18.2) have been published in Nature Medicine on June 3, 2024.

This trial included a dose-escalation stage (n = 15) and a dose-expansion stage in four different cohorts (total n = 83): cohort 1, satri-cel monotherapy in 61 patients with standard chemotherapy-refractory gastrointestinal cancers; cohort 2, satri-cel plus anti-PD-1 therapy in 15 patients with standard chemotherapy-refractory gastrointestinal cancers; cohort 3, satri-cel as sequential treatment after first-line therapy in five patients with gastrointestinal cancers; and cohort 4, satri-cel monotherapy in two patients with anti-CLDN18.2 monoclonal antibody-refractory gastric cancer. The primary endpoint was safety; secondary endpoints included efficacy, pharmacokinetics and immunogenicity. A total of 98 patients received satri-cel infusion, among whom 89 were dosed with 2.5 × 108, six with 3.75 × 108 and three with 5.0 × 108 CAR T cells. Median follow-up was 32.4 months (95% confidence interval (CI): 27.3, 36.5) since apheresis. No dose-limiting toxicities, treatment-related deaths or immune effector cell-associated neurotoxicity syndrome were reported. Cytokine release syndrome occurred in 96.9% of patients, all classified as grade 1–2. Gastric mucosal injuries were identified in eight (8.2%) patients. The overall response rate and disease control rate in all 98 patients were 38.8% and 91.8%, respectively, and the median progression-free survival and overall survival were 4.4 months (95% CI: 3.7, 6.6) and 8.8 months (95% CI: 7.1, 10.2), respectively. Satri-cel demonstrates therapeutic potential with a manageable safety profile in patients with CLDN18.2-positive advanced gastrointestinal cancer.
About Satri-cel
Satri-cel is an autologous CAR T-cell product candidate against the protein Claudin18.2 that has the potential to be the first-in-class globally. Satri-cel targets the treatment of Claudin18.2 positive solid tumors with a primary focus on gastric cancer/gastroesophageal junction cancer (GC/GEJ) and pancreatic cancer (PC). Ongoing trials include investigator-initiated trials (CT041-CG4006, NCT03874897), a confirmatory Phase II clinical trial for advanced GC/GEJ in China (CT041-ST-01, NCT04581473), a Phase I clinical trial for PC adjuvant therapy in China (CT041-ST-05, NCT05911217), and a Phase 1b/2 clinical trial for advanced gastric or pancreatic adenocarcinoma in North America (CT041-ST-02, NCT04404595). Satri-cel was granted Regenerative Medicine Advanced Therapy (RMAT) designation by U.S. FDA for the treatment of advanced GC/GEJ with Claudin18.2-positive tumors in January 2022 and was granted PRIME eligibility by the EMA for the treatment of advanced gastric cancer in November 2021. Satri-cel received Orphan Drug designation from the U.S. FDA in 2020 for the treatment of GC/GEJ and Orphan Medicinal Product designation from the EMA in 2021 for the treatment of advanced gastric cancer.
At present, there are still many clinical trials of new anti-cancer technologies in China seeking patients. Consultation on new drugs and technologies, you can contact Beijing South Region Oncology Hospital International Department.
Phone Number:4008803716
Email:myimmnet@163.com
References
1.https://www.cde.org.cn/main/xxgk/listpage/9f9c74c73e0f8f56a8bfbc646055026d
Post time: Feb-27-2025

