On January 16, 2025, China’s National Medical Products Administration (NMPA) has approved the New Drug Application (NDA) of limertinib for the treatment of adult patients with locally advanced or metastatic EGFR T790M-mutated non-small cell lung cancer (NSCLC).
About Limertinib
Limertinib is an orally-administrated, third-generation EGFR TKI with proprietary rights, approved by the China’s NMPA for the treatment of adult patients with locally advanced or metastatic EGFR T790M-mutated non-small cell lung cancer (NSCLC). A second NDA is under NMPA review for first-line treatment of adult patients with locally advanced or metastatic NSCLC carrying EGFR exon 19 deletions or exon 21 L858R mutations.
The drug demonstrated success in a multi-center, randomized, double-blind, controlled Phase 3 clinical trial comparing its efficacy and safety of to gefitinib in first-line treatment of patients with locally advanced or metastatic NSCLC harboring EGFR mutations. With the primary endpoint met, results will be presented at upcoming academic conferences or published in academic journals.
The regulatory decision was supported by data from a pivotal phase 2b trial (NCT03502850), This is a single-arm, open-label, phase 2b study conducted at 62 hospitals across the People’s Republic of China. Patients with locally advanced or metastatic NSCLC with centrally confirmed EGFR T790M mutations in tumor tissue or blood plasma who progressed after first- or second-generation EGFR tyrosine kinase inhibitors or with primary EGFR T790M mutations were enrolled.
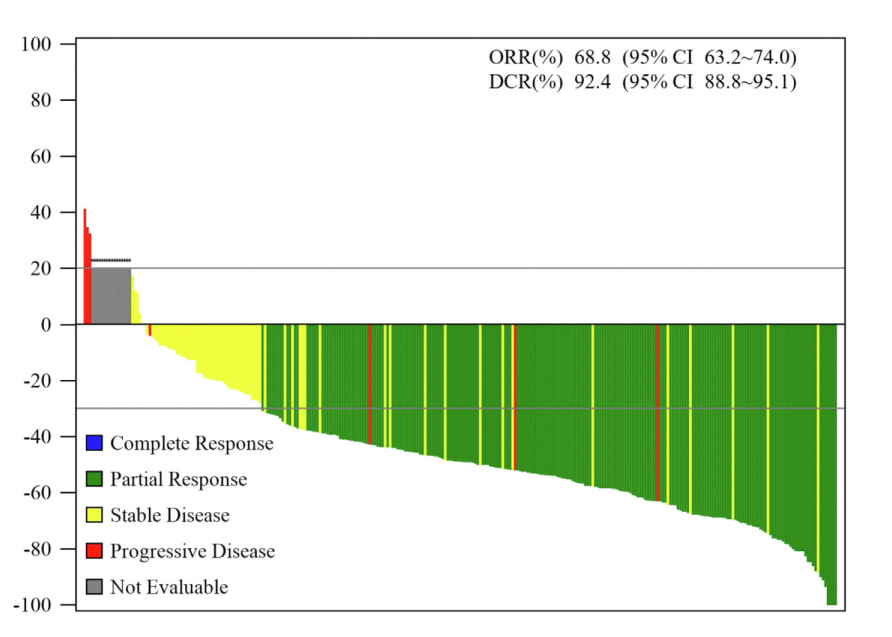
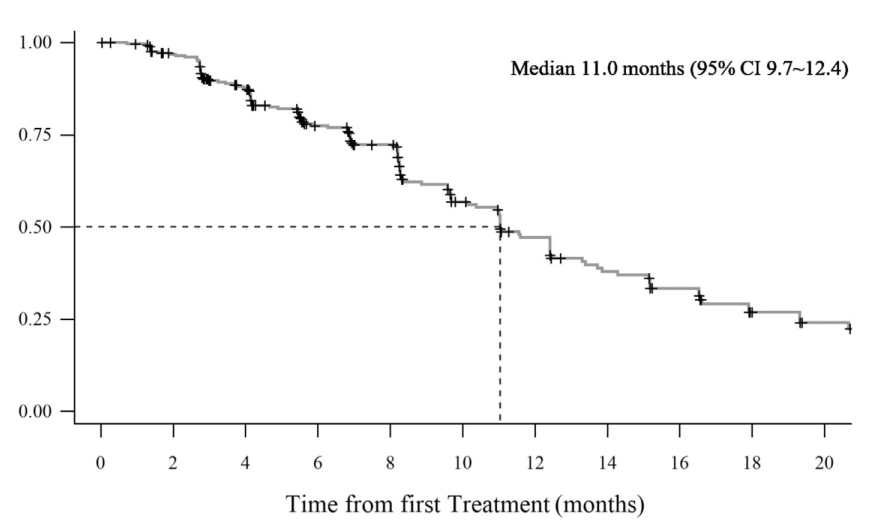
From July 16, 2019, to March 10, 2021, a total of 301 patients were enrolled and started the treatment of limertinib. All patients entered the full analysis set and safety set. By the data cutoff date on September 9, 2021, 76 (25.2%) remained on treatment. The median follow-up time was 10.4 months. On the basis of full analysis set, the independent review committee-assessed ORR was 68.8% and disease control rate was 92.4%. The median PFS was 11.0 months, median DoR was 11.1 months, and median OS was not reached. Objective responses were achieved across all prespecified subgroups. For 99 patients (32.9%) with central nervous system (CNS) metastases, the ORR was 64.6%, median PFS was 9.7 months (95% CI: 5.9–11.6), and median DoR was 9.6 months. For 41 patients who had assessable CNS lesion, the confirmed CNS-ORR was 56.1% and median CNS-PFS was 10.6 months .
In safety set, 289 patients (96.0%) experienced at least one treatment-related adverse event (TRAE), with the most common being diarrhea (81.7%), anemia (32.6%), rash (29.9%), and anorexia (28.2%). Grade ≥3 TRAEs occurred in 104 patients (34.6%), with the most common including diarrhea (13.0%), hypokalemia (4.3%), anemia (4.0%), and rash (3.3%). TRAEs leading to dose interruption and dose discontinuation occurred in 24.6% and 2% of patients, respectively. No TRAE leading to death occurred.
Conclusions
Limertinib (ASK120067) was found to have promising efficacy and an acceptable safety profile for the treatment of patients with locally advanced or metastatic EGFR T790M-mutated NSCLC.
At present, there are still many clinical trials of new anti-cancer technologies in China seeking patients. Consultation on new drugs and technologies, you can contact Beijing South Region Oncology Hospital International Department.
Phone Number:4008803716
Email:myimmnet@163.com
References
2.Jiangsu Aosaikang Pharmaceutical Co. Ltd. (ASK Pharm) (ask-pharm.com)
4.https://www.jto.org/article/S1556-0864(22)00267-2/fulltext
Post time: Jan-21-2025

