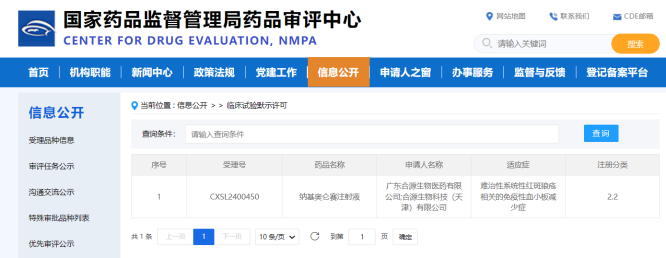
On October 11, 2024,China’s National Medical Product Administration (NMPA) has approved Investigational New Drug(IND)application for Inaticabtagene Autoleucel (CNCT19 Injection), a CD19-targeted autologous chimeric antigen receptor (CAR)-T cell therapy, to treat Immune thrombocytopenia of systemic lupus erythematosus (SLE – ITP). This is the first IND of Inaticabtagene Autoleucel approved for autoimmune disease in China.
Inaticabtagene Autoleucel is a CD19 CAR-T cell therapy product, featured with a unique CD19 scFv(HI19a)and advanced manufacturing processes. Inaticabtagene Autoleucel is the first proprietary CD19-targeted CAR-T product homegrown in China, and the first commercialized CAR-T cell therapy product for B-ALL in China . Inaticabtagene Autoleucel is expanding its application for several indications, including adult r/r B-ALL, r/r DLBCL, pediatric and adolescent r/r B-ALL, and various autoimmune diseases. Its New Drug Application (NDA) of treating Relapsed or Refractory Large B-Cell Lymphoma has been accepted by NMPA in September 2024.
SLE is a systemic autoimmune disease, usually leading to multiple organ damage. Abnormal B cells and autoantibody play a critical role in SLE pathogenesis. SLE could cause excessive platelet destruction, namely SLE-ITP. SLE-ITP occurs in approximately 16% of SLE Chinese patients, most of the SLE-ITP patients respond very poorly to steroids, immunoinhibitors, and biological drugs, and often suffer major organ bleeding risk,while their long-term survival is rather poor.
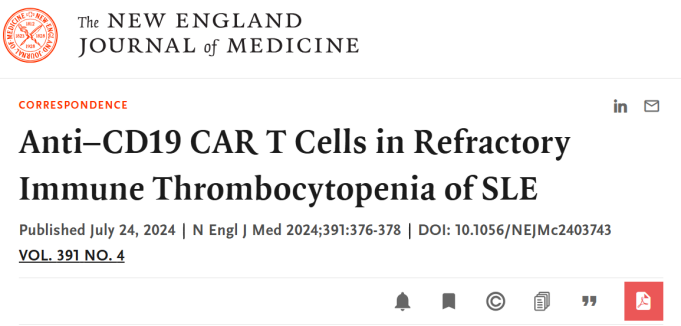
In July of this year, the prestigious 《The New England Journal of Medicine》 published a study “Anti-CD19 CAR T cells in refractory immune thrombocytopenia of SLE,” co-authored by Professor Zeng Xiaofeng and Professor Li Mengtao from the Rheumatology Department and Professor Zhou Daobin from the Hematology Department of Peking Union Medical College Hospital. The study showcased the long-lasting efficacy and safety of Inaticabtagene Autoleucel in treating SLE-ITP, highlighting the huge potential of CAR-T cell therapy.
A 38-year-old woman received a diagnosis of SLE in 2014 and presented with reversible proteinuria and arthritis and persistent thrombocytopenia. Previous treatments with high-dose glucocorticoids (methylprednisolone pulses), hydroxychloroquine, intravenous immune globulin, tacrolimus, cyclosporin A, sirolimus, rituximab, danazol, eltrombopag, and recombinant human thrombopoietin did not alleviate the thrombocytopenia . The platelet counts were maintained at 10,000 to 20,000 per cubic millimeter and eventually fell below 10,000 per cubic millimeter in July 2023.
At that time, she presented with purpura and ecchymoses on her limbs, oral blood blisters, and gingival bleeding. Her bone marrow smear showed active myeloproliferation and impairment of megakaryocyte maturation. Only low-dose prednisone (5 mg per day) and hydroxychloroquine (0.2 g per day) were administered in the month before the CAR T-cell infusion. Autologous fresh leukapheresis was used to produce inati-cel. A total of 500,000 CD19 CAR T cells per kilogram of body weight were administered after the patient had undergone lymphodepleting chemotherapy with fludarabine (at a dose of 25 mg per square meter of body-surface area) per day on days −5, −4, and −3 and cyclophosphamide (at a dose of 250 mg per square meter) on days −5 and −4 before CAR T-cell therapy.
After Inaticabtagene Autoleucel Injection, the patient’s platelet count continued to rise, and clinical complete response was achieved, and glucocorticoids and all immunosuppressants were discontinued. At present, the patient has basically returned to normal life.
At present, there are many other CAR-T clinical trials in China, and they are looking for patients. For consultation on new drugs and technologies, you can contact Beijing South Region Oncology Hospital International Department.
Phone Number:4008803716
Email:myimmnet@163.com
REFERENCES
1.http://www.juventas.cn/news.php?id=711
2.https://www.nejm.org/doi/full/10.1056/NEJMc2403743
Post time: Nov-08-2024