GC203 is a novel non-viral vector gene-modified TIL therapy developed leveraging Juncell Therapeutics’ proprietary DeepTIL® cell expansion platform and NovaGMP® gene modification platform. DeepTIL® enables TILs to be potent enough that no IL-2 combination will be required after infusion, and the intensity of pretreatment could be lower. NovaGMP® modifies T cells with a high efficiency comparable with the Lentiviral vector in an economic way. GC203 is engineered with self-associating membrane-bound interleukin-7, which can maintain the stemness of memory T cells, activate internal immune cells and avoid systemic toxicities.
Researchers tested GC203 on patients diagnosed with recurrent ovarian cancer, without the combination of IL-2 administration or aggressive lymphodepletion. The objective of the study was to determine the safety and efficacy of this treatment. A total of 20 patients were enrolled between 09/2021 and 01/2024 in the open-label single-center trial, in which 18 patients were evaluable. The evaluable patients were with an ECOG PS of 1 (55.6%) or 2 (44.4%) and received a median of 5 previous systemic therapies (range: 2-11). 9 (50%) patients had 3 or more tumor lesions. The median target lesion size was 57 (range: 13-146) mm.
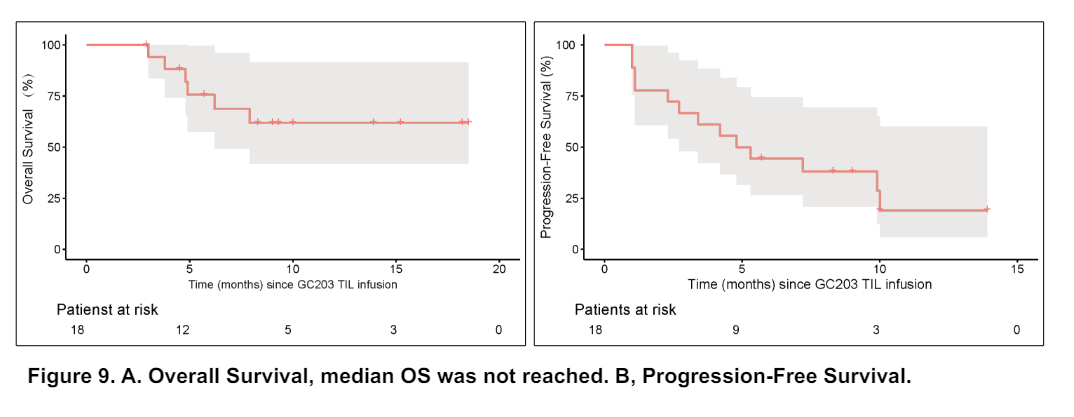
GC203 showed promising results in patients with recurrent or metastatic ovarian cancer. The safety profile was improved significantly compared with conventional TIL therapies, thanks to a low-intensity pretreatment and the elimination of IL-2 combination. Most of the Adverse Events (AEs) were grade 1 or grade 2 and could be alleviated or cured by symptomatic treatment. No grade 5 event occurred. By the cutoff date of 01/2024, the investigator-assessed Objective Response Rate (ORR) was 33.3% (95% CI: 16.3 – 56.3), including 11.1% Complete Response (CR). The Disease Control Rate (DCR) was 83.3% (95% CI, 60.8 – 94.2). And the 12-month Overall Survival (OS) rate was 68.8% (95% CI: 49.3 – 95.9). The median OS was not mature as data cut-off.
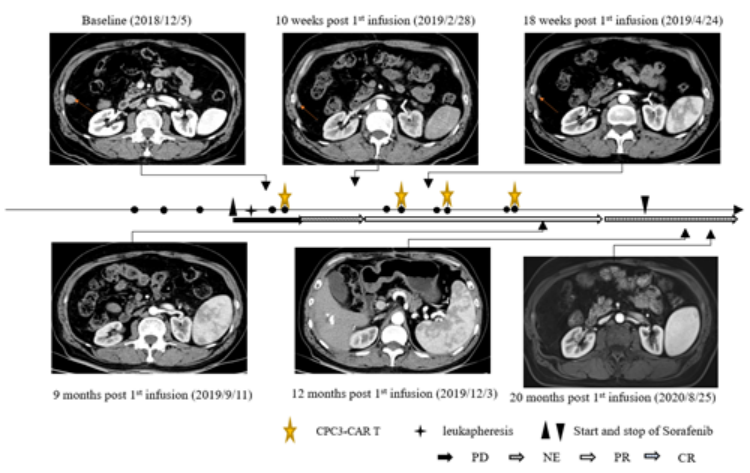
In 2023, Ms. H was admitted to the hospital in 2023 because of groin and waist pain,Upon examination, multiple lymph nodes were found to be enlarged in the retroperitoneum, groin, and other areas,confirming her diagnosis of high-grade serous ovarian cancer.So Ms. H underwent chemotherapy, surgery, immunotherapy, etc., but the effect was not ideal, and the tumor actually recurred.
Therefore,Ms. H participated in the clinical trial of TIL cell therapy GC203, and after one month of treatment with GC203, the tumor shrank by 37.3%.
Conclusions: Infusion of GC203 has acceptable safety and potent efficacy in recurrent OC patients who have limited treatment optio.
At present, there are still many clinical trials of new anti-cancer technologies in China seeking patients. Consultation on new drugs and technologies, you can contact Beijing South Region Oncology Hospital International Department.
Phone Number:4008803716
Email:myimmnet@163.com
References
1.https://www.juncell.com/academic-achievements
2.https://www.juncell.com/company-news-76
3.https://ascopubs.org/doi/10.1200/JCO.2024.42.16_suppl.5552
4.http://myimm.net/mianyizhiliao/2037.html
Post time: Dec-11-2024