Recently,GenFleet announced the latest phase II data of KROCUS study, fulzerasib (GFH925, KRAS G12C inhibitor) in combination with cetuximab for first-line non-small cell lung cancer (NSCLC) treatment,in a late-breaking abstract at the mini oral presentation of the 2025 European Lung Cancer Conference (ELCC) annual meeting.
Fulzerasib the first China-developed KRAS G12C inhibitor that has its NDA submission accepted and granted with Priority Review Designation by NMPA. Fulzerasib also received Breakthrough Therapy Designations this year for treating advanced KRAS G12C-mutant NSCLC patients that have received at least one systemic therapy and CRC patients who have received at least two systemic therapies.RAS protein family can be divided into KRAS, HRAS and NRAS categories. KRAS mutations are detected in nearly 90% of pancreatic cancer, 30-40% of colon cancer, and 15-20% lung cancer patients. The occurrence of KRAS G12C mutation subset is more frequently observed than those with ALK, ROS1, RET and TRK 1/2/3 mutations combined. GFH925 is a novel, orally active, potent KRAS G12C inhibitor designed to effectively target the GTP/GDP exchange, an essential step in pathway activation, by modifying the cysteine residue of KRAS G12C protein covalently and irreversibly. Preclinical cysteine selectivity studies demonstrated high selectivity of fulzerasib towards G12C. Subsequently, fulzerasib effectively inhibits the downstream signal pathway to induce tumor cells’apoptosis and cell cycle arrest.
A total of 47 previously untreated advanced KRAS G12C-mutant NSCLC patients were treated with fulzerasib in combination with cetuximab (fulzerasib 600mg BID + cetuximab 500 mg/m2 Q2W) as of Jan 14, 2025.
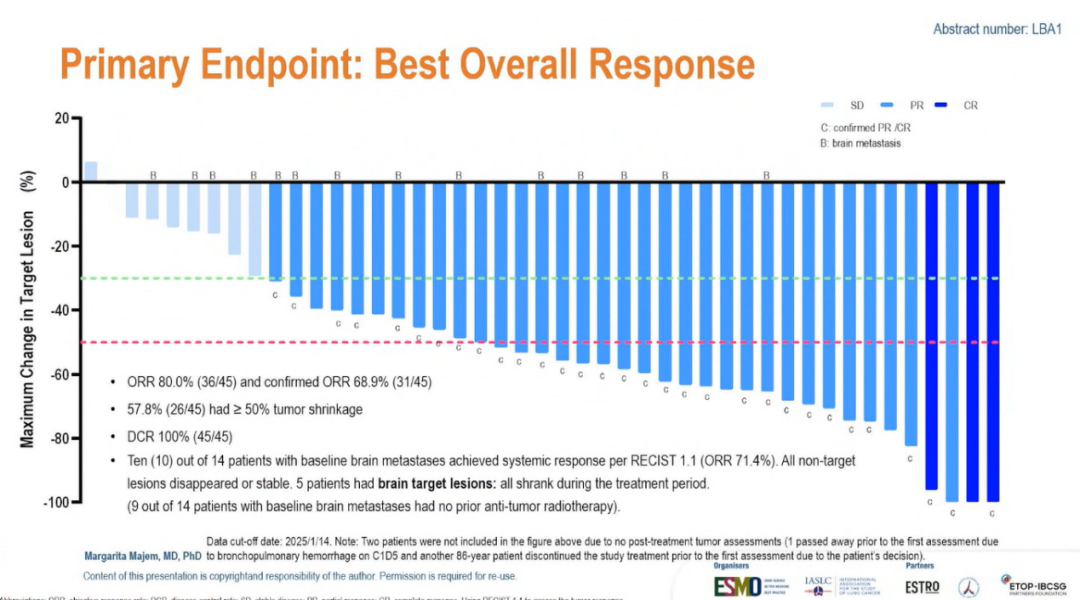
Efficacy: As of data cutoff date, among the 45 patients who received at least one post-treatment tumor assessment, the ORR was 80% and DCR was 100%; 57.8% had ≥ 50% tumor shrinkage. 16 patients (34%) had brain metastasis; among the 14 brain-metastatic patients that received at least one post-treatment tumor assessment, the ORR per RECIST 1.1 was 71.4%. The median duration of response (DoR) was not reached yet, and 24 patients were still on treatment with a median follow-up of 10.1 months. The mPFS was 12.5 months and the mOS was not reached.

Safety: As of data cutoff date, the combination therapy presented a favorable safety/tolerability profile. TRAEs occurred in 87.2% of patients and the majority of the TRAEs were graded 1-2; 14.9% of patients experienced at least one grade 3 TRAEs; no grade 4-5 TRAEs. 2 patients had treatment-related serious adverse events (TRSAE) and the TRSAEs were assessed to be merely related with cetuximab; 3 patients experienced TRAEs, unrelated to fulzerasib, leading to dose discontinuation. KROCUS demonstrated a relatively low occurrence of dose discontinuation or reduction among different first-line G12C-mutant NSLCL combo studies. No new safety signals were identified compared with fulzerasib or cetuximab as single agent.
At present, there are still many clinical trials of new anti-cancer technologies in China seeking patients. Consultation on new drugs and technologies, you can contact Beijing South Region Oncology Hospital International Department.
Phone Number:4008803716
Email:myimmnet@163.com
Post time: Apr-15-2025
